Data analytics have thoroughly disrupted industry after industry, and the healthcare sector stands to gain more than most. Data technologies have many applications in the industry, with predictive analytics in clinical trials being one of the most advantageous.

What is clinical trial data analytics?
Clinical trial analytics uses machine learning algorithms to gain new insight into clinical trial data. These clinical trials play a critical role in drug development, product testing and patient health, so pharmaceutical companies face high standards. Data-driven insights make it far easier to meet those expectations.
How medical businesses use clinical trial analytics can vary. In some cases, they use predictive analytics services to model how various patient classes would react to certain drugs. These insights can guide real-world trials by providing baseline expectations and helping researchers refine their products before moving to clinical trials.

Source: Unsplash
Regulatory bodies require clinical trials on animals to ensure drugs aren’t toxic before testing them on humans. Predictive analytics in these clinical trials can analyze results from animal tests to predict how simial situations may play out with human patients.
In other cases, medical organizations use clinical trial data analytics to find study participants. Artificial intelligence (AI) algorithms scan electronic health records (EHRs) to find people who may be ideal for testing a given medication or product.
How data analytics improve clinical trials
Regardless of the specific use case, predictive analytics in clinical trials has many benefits. Here are a few of the most significant advantages healthcare companies can realize from implementing these technologies.
Streamlining the trial process
One of the biggest advantages of clinical trial analytics is that they speed up the clinical trial process. Drug development projects must go through four clinical trial phases, some of which can take up to four years and require thousands of participants. While clinical trial predictive analytics don’t let companies skip these phases, they can help them go through them faster.
Finding study participants is one of the most time-consuming yet non-value-adding parts of the process. Volunteers must be willing, cover enough different demographics and include certain health conditions for trial results to be accurate. Finding and contacting enough participants to fit all those categories is challenging by manual means, but AI can do it in minimal time.

Predictive analytics in clinical trials can further streamline the process by predicting results. AI models could predict side effects, drug efficacy, medication interactions and more before moving to another trial phase. These insights can appease regulatory bodies and investors sooner despite having fewer real-world examples, helping drugs move through the phases more quickly.
Minimizing clinical trial costs
Clinical trial predictive analytics also helps minimize the costs of drug or medical product development. It can cost more than $2 billion to develop a drug, much of this comes from trial-related expenses. Clinical trial analytics software helps by shortening timelines, preventing errors and reducing administrative burdens.

Source: Unsplash
Because machine learning and AI streamline clinical trial phases, these companies spend less on ongoing costs per drug. These shortened timelines also lead to quicker returns on investment (ROI), helping account for any expenses they do incur. Early promising results from analytics models may help attract more investment, too.
Data analytics also reduces costs by predicting trial outcomes and adverse events before moving to real-world trials. These predictive models can highlight adverse events and reactions before they occur, letting drug developers address these issues before testing medications on people. Consequently, clinical trials will likely involve fewer unforeseen issues, minimizing remediation costs.
On a smaller scale, data analytics in clinical trials improves productivity by automating research and administrative tasks. As a result, researchers can accomplish more at once, making the most of their ongoing expenses.
Improving clinical outcomes
Another impressive advantage of clinical trial predictive analytics is the improvement of patient outcomes. Because these algorithms can predict potential issues with drugs or products before they move to human trials, they prevent dangerous side effects. Even before that stage, predictive modeling can reveal how people may react to various drugs, helping researchers find the most effective treatments earlier.

Source: Unsplash
Machine learning algorithms can test millions of possibilities in a fraction of a second. Pharma companies can use that analytical speed to discover new drugs or test them in various scenarios, highlighting the most promising candidates. By the time they bring these medicines to human trials, they’ll have the assurance that they’ll be highly effective.

Big data analytics for clinical trials
Predictive analytics in clinical trials is beneficial at virtually any scale, but Big data takes these benefits further. Big data analytics for clinical trials works the same but uses far larger datasets to make predictions. These datasets exceed the capabilities of traditional data management solutions in three key areas:
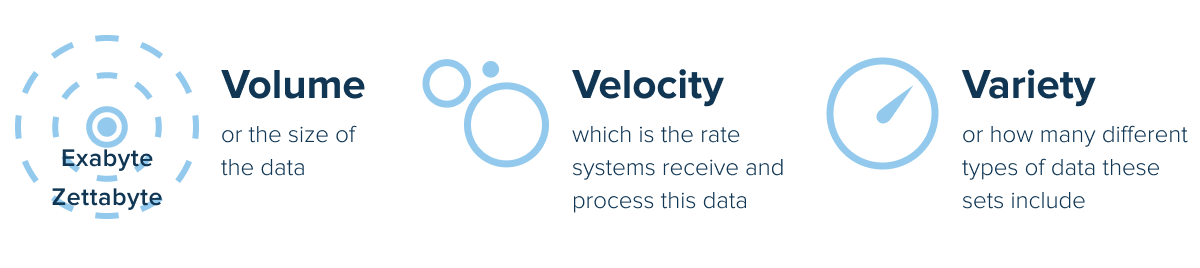
These three characteristics give Big data analytics in clinical trials an edge over conventional analytical processes. With more information coming in at higher rates and covering more diverse types, analytical models can make more accurate predictions. The third factor, variety, is particularly important here, as clinical trial researchers must ensure their medication works for people of all demographics and conditions.
Big data makes predictive analytics in the pharmaceutical industry faster and more reliable. While it may be more difficult to gather and analyze these datasets, those benefits are difficult to overlook. With the right resources, medical organizations can use them to take their predictive models to the next level.
Examples of predictive analytics in healthcare
While predictive analytics in business is now a fairly widespread phenomenon, its application in healthcare is still relatively new. Despite that recency, real-world case studies already demonstrate how these data technologies can improve clinical trials.
Some of the most recent and impressive use cases of data analytics for clinical trials come from the COVID-19 pandemic. The developers of the Johnson & Johnson COVID vaccine used machine learning to predict upcoming surges that would serve as ideal testing grounds. As a result, they could find test groups for their vaccine trials earlier, speeding development.

Source: Unsplash
Moderna also used AI data analytics to develop its COVID-19 vaccine. Researchers at Moderna used predictive models to test roughly 1,000 mRNA sequences a month to find the ideal candidate for its vaccine. Consequently, the company was able to push out a highly effective shot in minimal time.
Overview: predictive analytics in clinical trials
Like many healthcare IT solutions, predictive analytics in clinical trials requires some specific considerations. No technology is perfect, and medical companies must recognize and account for predictive analytics’ limitations to use it effectively. Here are a few of the most significant of these considerations for clinical trial predictive analytics.
Privacy and cybersecurity
One of the most important factors to consider with these technologies in medicine is their security. Implementing machine learning solutions requires a lot of data. In the medical sector, this information is often highly sensitive, so companies must go to great lengths to secure it.
Privacy regulations like the Health Insurance Portability and Accountability Act (HIPAA) hold pharmaceutical companies to high standards of cybersecurity and data privacy. Collecting EHRs and other sensitive data to hold in one place for data analytics complicates that. With so much health information in one place, these machine learning databases may be tempting targets for cybercriminals.

Source: Unsplash
Given the size of these databases, just one breach could give hackers access to potentially thousands of patients’ medical histories. On top of affecting the privacy of these people, that would incur high fees and mediation costs for the company.
In light of these risks, clinical trial databases should employ advanced security measures like zero-trust architecture and automated monitoring. Scrubbing data of any personally identifiable information (PII) before feeding it to predictive algorithms will also help maintain patient privacy.
Algorithm bias
Medical companies using data analytics in clinical trials must also consider AI bias. While this technology itself isn’t innately prejudiced, deep-rooted historical and cultural biases can seep in during training that machine learning then expands upon.
Developers may develop predictive models that aren’t as effective when predicting health outcomes in people of color by unintentionally feeding it EHRs from mostly caucasian patients. These errors can be difficult to catch, but if data scientists don’t recognize and fix them early, machine learning models could quickly become biased.
To stop this from happening, pharmaceutical companies must first recognize how these biases can arise. Then, they should routinely review their data collection and model training processes to ensure they don’t accidentally create these prejudices.
Technical expertise
A lack of expertise with these technologies is another potential obstacle. Medical professionals are highly knowledgable, but they’re not data science experts. Consequently, they may not know how to build and implement analytics models effectively.
Thankfully, this issue is a relatively easy one to overcome. Medical organizations can offer upskilling and reskilling programs to teach clinical trial researchers about data science concepts and best practices. Alternatively, companies can outsource the most technically complex analytics processes to firms that are experts in the field.
Source: Unsplash
Other uses of predictive modeling in healthcare
If medical companies carefully consider these factors, predictive modeling can be a revolutionary tool. Unsurprisingly, these extensive benefits can translate to other parts of the healthcare sector, too.

Medical researchers can also use predictive analytics to monitor global health. Because machine learning is skilled at recognizing patterns to predict similar situations in the future, it’s ideal for highlighting areas where outbreaks may occur. There’s already real-world evidence for these use cases’ potential, as one algorithm predicted COVID-19 nine days before the World Health Organization released its initial statement on the virus.
As this technology develops, more use cases and benefits will emerge, too. The healthcare industry could improve across the board from implementing data analysis tools.
Predictive analytics to revolutionize clinical trials
Predictive analytics in clinical trials may be a fairly new practice, but its potential is substantial. If medical companies can develop and implement these tools carefully, they could revolutionize the industry. Clinical trials will take less time, be more effective, cost less money and save more lives.
Author bio
April Miller is a senior writer with more than 3 years of experience writing on AI and ML topics.
Leverage predictive analytics for clinical trials
Need help with pharma solutions development? Contact us, and we’ll set up a call to discuss your business needs and how we can apply technology to it.